Abstract
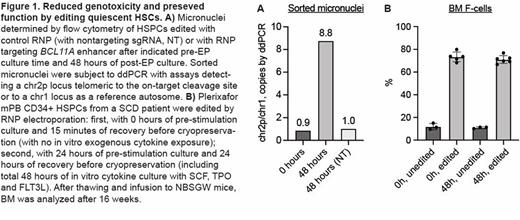
Therapeutic gene editing of HSCs promises to cure inherited blood disorders. However, current ex vivo gene editing protocols may be associated with genotoxic potential and complex in vitro cell manufacturing procedures. Base and prime editing reduce double strand breaks (DSBs) but are generally less efficient and might have unique genotoxic potential (e.g. untargeted deaminase activity). Here we test the hypothesis that therapeutic gene editing with targeted endonucleases disrupting HbF-repressing elements for b-hemoglobinopathies may be made safer, simpler, and at least as effective by focusing the editing on nondividing cells. Genotoxic risks of DSBs appear to be concentrated in cycling cells. For example, Cas9-induced DSBs can result in long deletions at the on-target site that appear to be mainly the result of unidirectional extensive resection selectively active in S/G2 cell cycle phases1. In addition, DSBs must be resolved prior to mitosis to prevent chromosome mis-segregation leading to copy number variation and cytoplasmic micronuclei2,3. We measured the cell cycle state of mobilized PB CD34+ HSPCs immediately after collection, or after 24 or 48 hours of culture with cytokines (SCF, TPO, and FLT3L each at 100 ng/ml). 100% of cells were in G0/G1 at 0 hours, 94.6%±1.5% at 24 hours, and 76.7%±2.6% at 48 hours. We electroporated CD34+ HSPCs with 0, 24, or 48 hours of cytokine culture before performing 3xNLS-HiFi-SpCas9:sgRNA ribonucleoprotein (RNP) electroporation targeting the BCL11A erythroid enhancer. We employed a flow cytometry assay to quantify micronuclei in edited HSPCs. The fraction of micronuclei was increased in cells electroporated with Cas9:sgRNA RNP following 24 or 48 hours of cytokine stimulation but not in unstimulated cells. We performed ddPCR on sorted nuclei and micronuclei using assays specific to distal chromosome 2p (telomeric to the BCL11A cleavage site) and to chr1 as a reference autosome. We found ~9-fold enrichment of chr2p in micronuclei after 24 or 48 hours of pre-stimulation cytokine culture prior to gene editing, but strikingly did not observe any enrichment of chr2p in micronuclei in cells edited without pre-stimulation culture, suggesting that chromosome mis-segregation and unwanted downstream consequences could be avoided by editing quiescent HSCs (Fig. 1A). In addition, we developed ddPCR assays to measure genome editing outcomes after editing the BCL11A enhancer with paired cleavages (at the +58 and +55 enhancers) separated by 3-kb. These assays measure: 1) programmed 3-kb deletions, 2) programmed 3-kb inversions, or 3) short indels or unedited alleles. Any missing signals (genomic copies fewer than found on a reference nontargeted autosome) may represent long deletions or rearrangements. Editing cells without pre-stimulation culture was associated with undetectable missing signals (inferred long deletions and rearrangements) as compared to editing after 24 or 48 hours of pre-stimulation culture. We compared our standard protocol of 24 hours of pre-stimulation cytokine culture and 24 hours of post-electroporation cytokine culture to a protocol in which CD34+ HSPCs were electroporated immediately after thawing and then cryopreserved 15 minutes after electroporation, without any cytokine culture, before infusion to NBSGW mice. After 16 weeks, HbF levels were potently induced to similar levels after gene editing in recipients of HSPCs without and with cytokine culture (Fig. 1B). Overall human engraftment was similar for all groups, as was multilineage repopulation. Gene edit frequencies were similar for recipients of HSPCs without and with cytokine culture. We observed comparable editing and engraftment potency of HSPCs without as compared to with cytokine culture across mobilization type (G-CSF or plerixafor), donor disease status (healthy and SCD), and sgRNA target sequence. Together, these results suggest that editing HSPCs ex vivo without in vitro culture: avoids unintended on-target genotoxicity potential, including production of micronuclei, long deletions, and rearrangements; simplifies protocols, eliminating time, cost, and complexity; and preserves gene editing efficiency and HbF induction and HSC repopulation potency. Editing nondividing cells selectively could be a straightforward modification to improve ex vivo editing as well as to advance future efforts for safe and potent in vivo editing of HSCs.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal